Nickel catalyst can replace expensive palladium in industrial chemistry
Source: interestingengineering
Author: @IntEngineering
Published: 7/4/2025
To read the full content, please visit the original article.
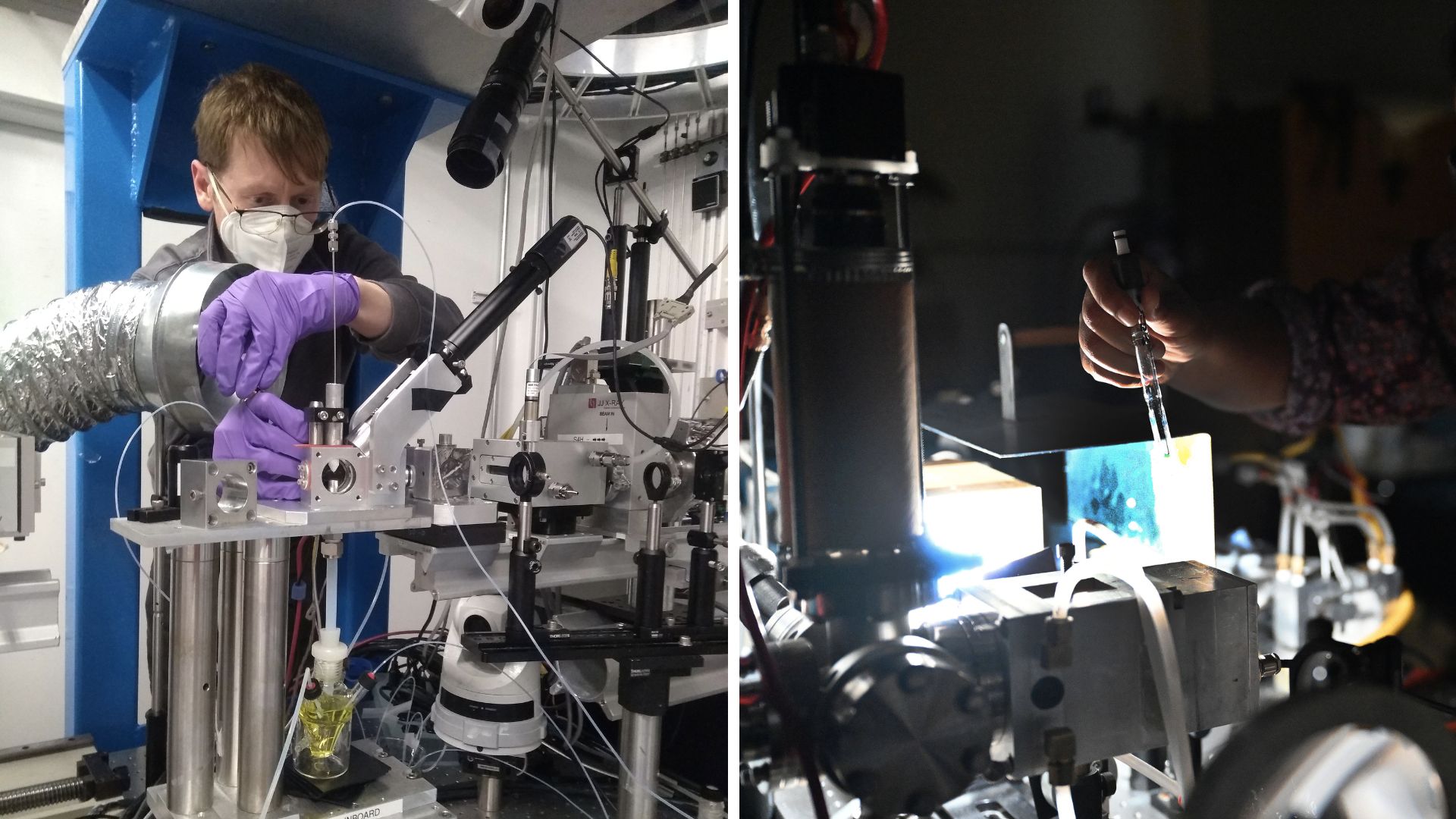
Read original articleUS Department of Energy scientists have revealed how nickel-based catalysts, activated by light, can effectively replace expensive palladium catalysts in industrial chemistry. Unlike palladium, which requires high heat, nickel’s reactivity can be driven by light, enabling milder reaction conditions and expanding the range of possible chemical reactions. This breakthrough is significant because nickel is abundant and much cheaper—costing about 50 cents per ounce compared to palladium’s nearly $1,000 per ounce—making it a promising alternative for large-scale chemical manufacturing in pharmaceuticals, agriculture, and electronics.
The research focused on nickel dihalides, where light exposure breaks the bond between nickel and halide ions, lowering nickel’s oxidation state and activating it for catalysis. A key discovery was that the freed halide ion interacts with the solvent to form a stable intermediate, preventing deactivation of the nickel catalyst by stopping nickel atoms from directly interacting. This insight into the light-driven catalytic mechanism was achieved using advanced spectroscopic techniques at national laboratories, including
Tags
materialsnickel-catalystpalladium-replacementindustrial-chemistrylight-driven-catalysischemical-reactionsenergy-efficient-processes